My Projects
Topic: Nanofiltration
Title: Recovery of Etching Liquor from Chemical Milling
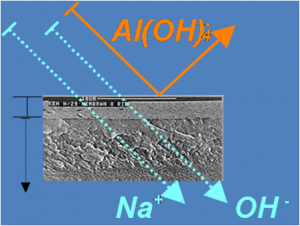
The image shows a cross section of a nanofiltration membrane.
The assembly consists of a selective layer with 0.2 µm thickness
supported by a non-selective backup layer.
What one doesn’t see:
The selective layer permits permeability up to about 100 u, i.e. atomic mass units. Thus, simple compounds and ions can pass, whereas larger molecules and complex clusters are retained. Thereby, an osmotic pressure occurs at the membrane, which has to be compensated by a mechanical overload for the generation of a material flow. By the backup layer, a pressure load up to about 40 bars becomes possible. Furthermore, the membrane material allows operating conditions up to 60°C and pH 15.
What’s behind it:
Nanofiltration enables the in-situ separation of the products from a chemical reaction. Thereby the concentration of the reactants remains almost constant and the process can be controlled precisely and operated permanently.
What you can obtain from it:
Caustic liquids are used for precision machining or the production of a base material. For instance, caustic soda is largely used in the aluminum industry to treat products or to produce mineral aluminate crystals. However, during the process, the concentration of the reactants is permanently changing and hence the reaction rate varies, which requires a steady control of the process and a steady readjustment of the operating parameters. Furthermore, the whole procedure needs a regular exchange of the complete substance – as well as its removal and reconditioning. This causes an increased effort for handling, off time and removal fees. Therefore, an integration of a nanofiltration provides improved results with less effort. In particular, chemical milling is a process, where the aluminum surface is substantially removed by caustic soda. Thereby, a geometrically precise treatment up to about 10 µm excavation depth becomes feasible on very large surfaces up to several square meters width. Furthermore, the process avoids structural harm by mechanical forces, as required for bent and structured aluminum sheets of aircraft bodies, for example. The efforts for process control, off time and removal, are basically diminished by an integration of nanofiltration since the dissolved aluminum is discharged as a concentrate during the process. In a similar way, specific aluminate crystals become feasible by form and size when the operating conditions are gauged by a suitable proportion of the reactants.
My related publications:
Method and Device for Operating Milling Baths, patent US 6454958 and WO 1998041672 A1 (1998)